News
Read all the latest news from the Medicines for Children team
Filter by...
-

Helping your child to swallow tablets – new resources from Kidzmed
Being able to swallow tablets is an important life skill that children need to learn. The Kidzmed programme has developed a guide to help children and young people learn to swallow tablets using six simple steps.
Read more
-

Benji the bear talks about drooling
Drooling is a common problem in children with cerebral palsy and other conditions that affect the nerves and muscles in the mouth. Drooling can be distressing for children and their families; however treatments are available. Medically, drooling is known as sialorrhoea.
Read more
-

Medicines for Children’s 2024 highlights
2024 was another busy and productive year for the Medicines for Children team. As we look ahead to 2025, it is important to reflect on some of the main achievements from the last 12 months, and to take this opportunity to thank the healthcare professionals and families who gave up their time and expertise to help us to develop new materials for the website.
Read more
-

Celebrating 30 years of our programme partner, the NPPG
The Medicines for Children team were invited to meet the paediatric pharmacists and technicians from the Neonatal and Paediatric Pharmacy Group at their 30th annual conference in November 2024. The NPPG is a valued partner of Medicines for Children since the programme started back in 2006.
Read more
-

Introducing new acknowledgment certificates for developing medicines information
Medicines for Children has introduced official letters of acknowledgment for health professionals contributing to our medicine information leaflets for families. These documents can be used as evidence for personal development portfolios or CPD. This initiative seeks to support contributors who volunteer their time to Medicines for Children, ensuring more families receive clear and accurate information on their children's medicines. Backdated certificates are offered to those who have participated previously.
Read more
-

Concerns raised about overuse of ‘PPIs’ in babies
Research published over the summer raised concerns that some babies may be receiving unnecessary treatment for regurgitation (reflux) using a type of medicine called proton pump inhibitors, shortened to PPIs. These include omeprazole, esomeprazole and lansoprazole. This Medicines for Children news item explains what PPIs are and whether they are helpful in babies.
Read more
-
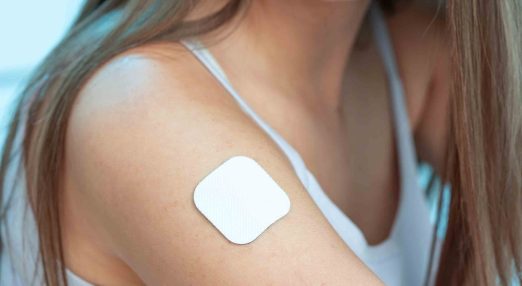
New advice to address safety concerns when using clonidine patches for dystonia
New guidance has been issued to address safety concerns regarding the use of clonidine patches for children with dystonia, emphasising proper patch application and the importance of following dosing instructions. Parents are advised to ensure patches are applied correctly and have a backup plan for switching to liquid medicine if patches are unavailable due to supply issues.
Read more
-

How can we improve Medicines for Children?
The Medicines for Children team needs your help! We aim to provide reliable and accessible information on a range of medicines given to children. We want to hear from parents, carers, health care professionals - anyone who uses Medicines for Children - to understand if our information has been helpful, and what we could do to improve.
Read more
-

Changes to potassium supplements
Medicines for Children has updated its information on how to give potassium using effervescent tablets. This is because supplies of the liquid potassium medicine called 'Kay-Cell-L' are likely to be limited over the next few months. As an alternative, children may be prescribed effervescent tablets and parents/carers will need to know how to give this medicine.
Read more
-

Two of the Medicines for Children board given awards for their significant contributions to children’s health
We are delighted and proud to announce that two members of the Medicines for Children board – Steve Tomlin and David Tuthill – have been made Honorary Fellows of the Royal College of Paediatrics and Child Health (RCPCH).
Read more
-

Childhood vaccinations – we want to hear your views!
The Royal College of Paediatrics and Child Health is undertaking a programme of work to look into the declining rates of childhood vaccinations in the UK. They want to hear from a range of health professionals and parents/families to understand their views on the barriers they may have faced in getting children vaccinated.
Read more
-

Vacancy to join the Medicines for Children programme board
The Medicines for Children team is looking for an RCPCH trainee to join the programme board, to take the newly vacant role of RCPCH trainee representative.
Read more
